Clinical FDA CE Blood Sugar Detection Bluetooth Automatic Blood Glucose Monitor with Test Strips Transmission Protocol - China Glucometer, Glucose Meter | Made-in-China.com
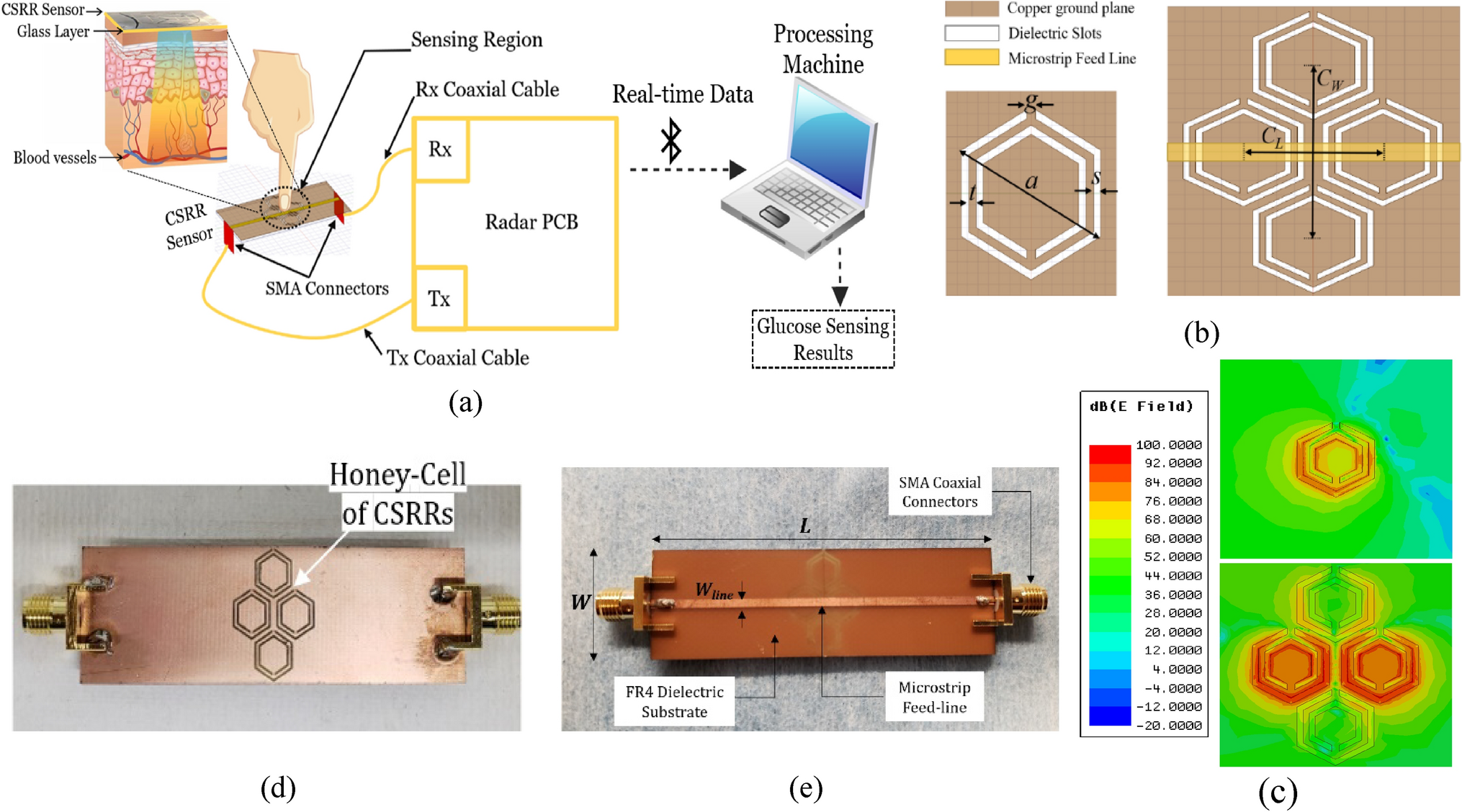
Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: novel design utilizing a four-cell CSRR hexagonal configuration | Scientific Reports

Landscape of Continuous Glucose Monitoring (CGM) and Integrated CGM: Accuracy Considerations | Diabetes Technology & Therapeutics
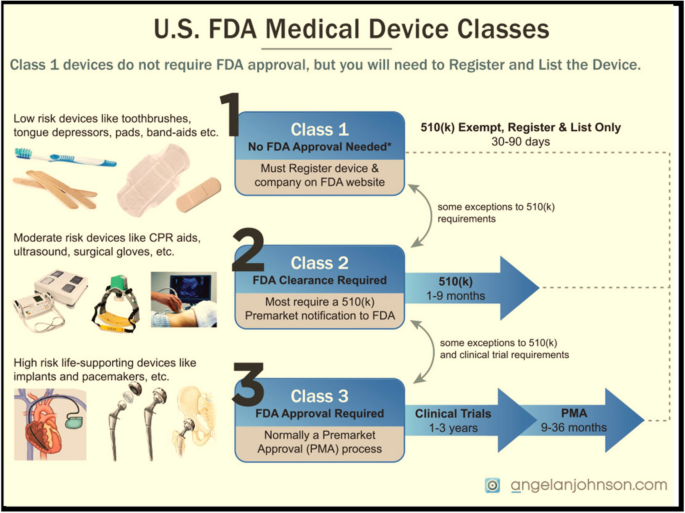
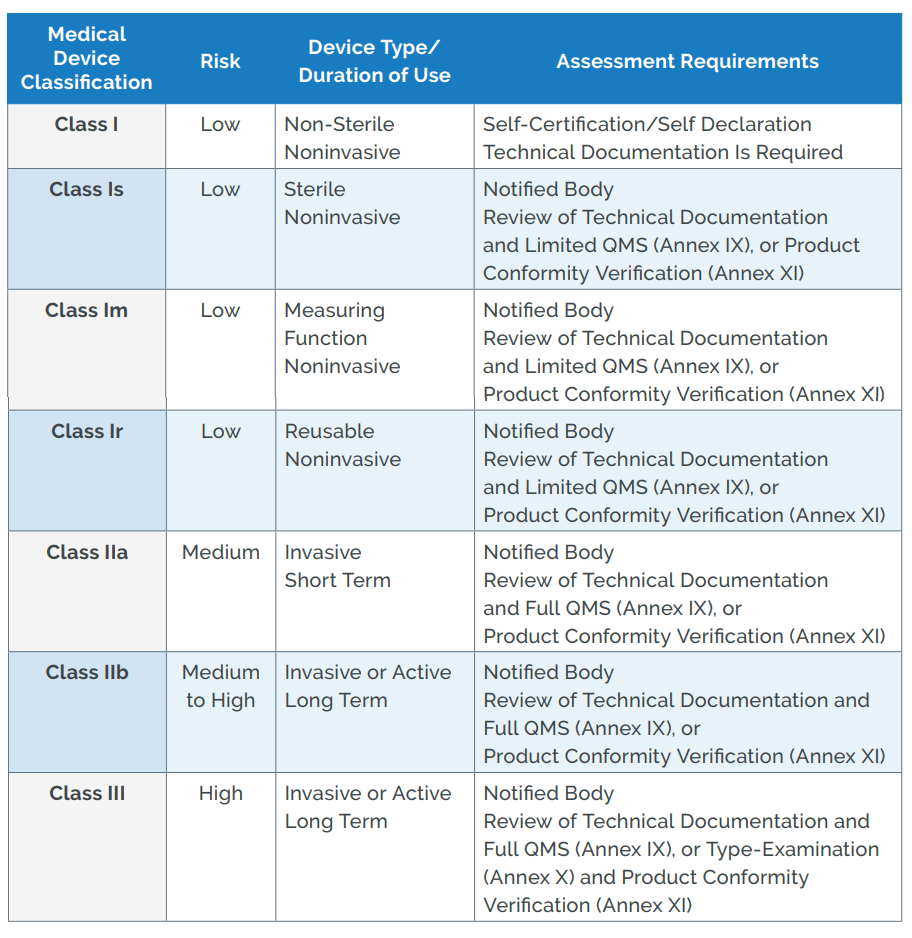
A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity | Biomedical Materials & Devices
US FDA and EU Risk Classification of Medical Devices | Emergo by ULFDA and EU Medical Device Risk Classification: Impact on Human Factors

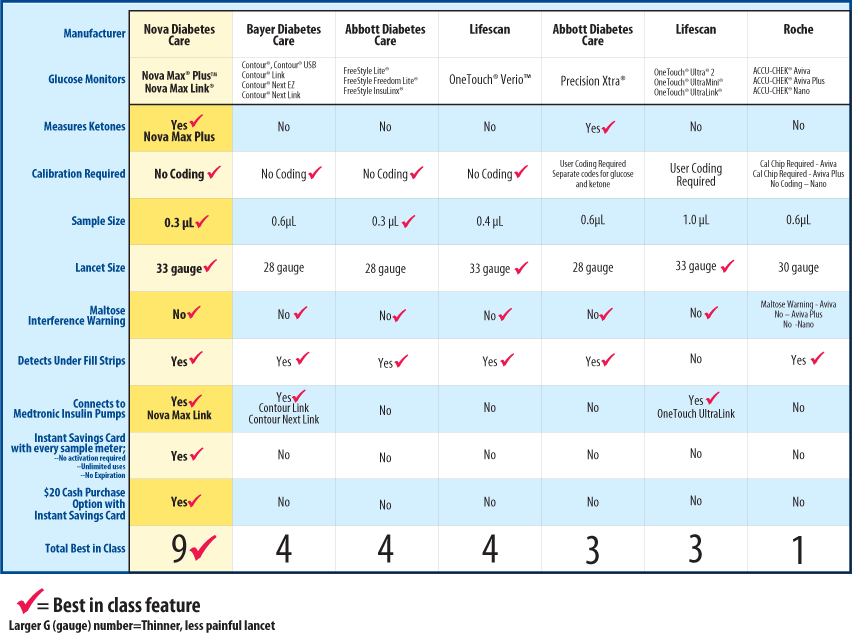
Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer

Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer

FDA Issues Final Orders to Reclassify Blood Lancet Devices into Class II and Class III Devices; Orders Include a PMA Requirement for Class III Blood Lancets - Registrar Corp
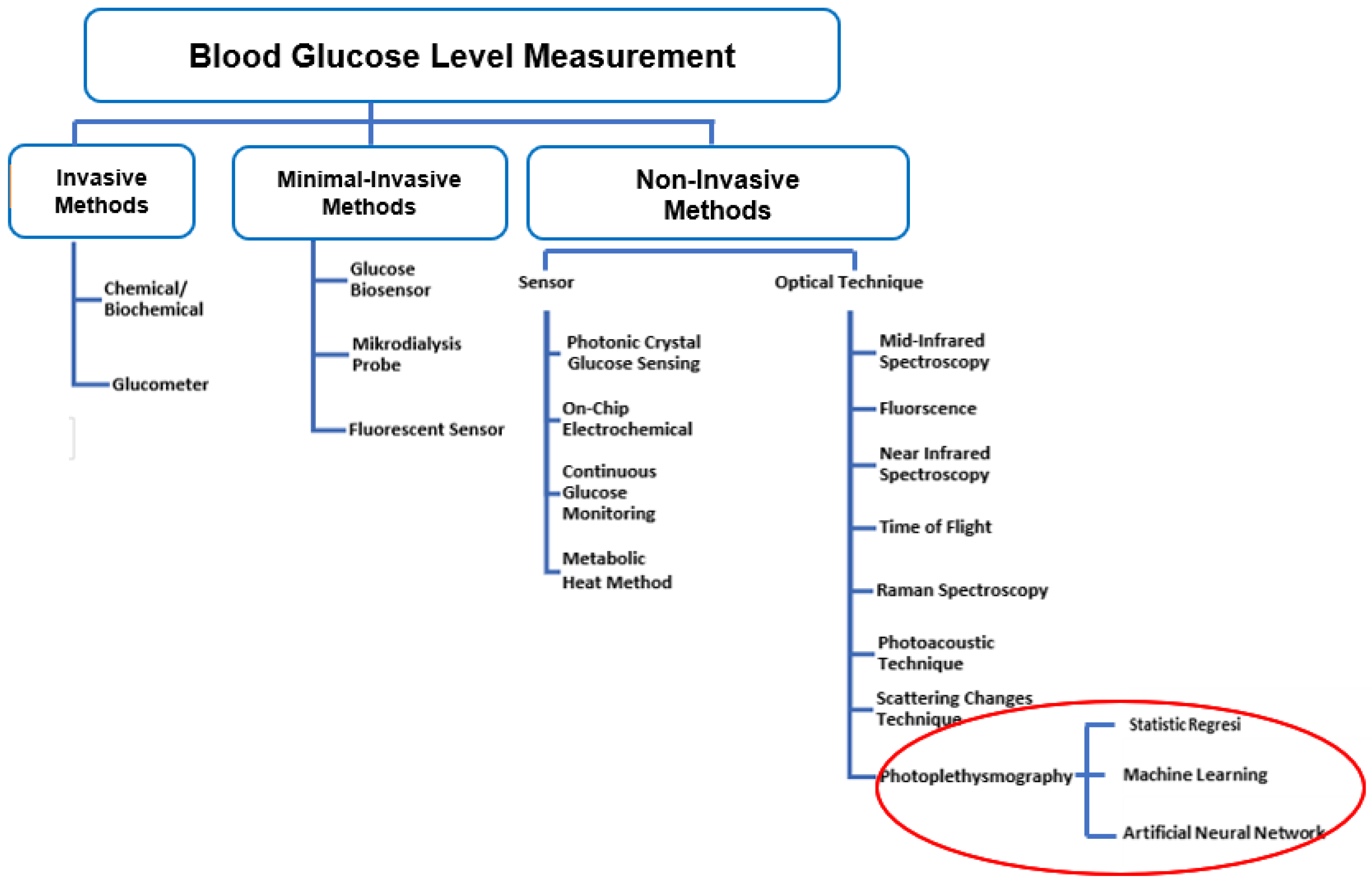
Information | Free Full-Text | Non-Invasive Classification of Blood Glucose Level for Early Detection Diabetes Based on Photoplethysmography Signal

Monitoring Technologies- Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control - Endotext - NCBI Bookshelf

Clinical FDA CE Blood Sugar Detection Bluetooth Automatic Blood Glucose Monitor with Test Strips Transmission Protocol - China Glucometer, Glucose Meter | Made-in-China.com